 |
 |
- 发展协作整合赛多利斯Stedim生物技术的BIOSTAT®STR生物反应器和Repligen的XCELL™ATF细胞保留控制技术
- 从强化细胞培养简化,可扩展解决方案的客户受益
哥廷根,德国和沃尔瑟姆,MA,USA,2018年9月4日/美通社B3C / -金宝搏官网mg赛多利斯Stedim生物技术(SSB)(欧洲证券交易所:DIM),国际领先的供应商,为生物医药产业,Repligen公司(NASDAQ:RGEN), a global life sciences company focused on bioprocessing technologies, have entered into a collaboration agreement to integrate Repligen’s XCell™ ATF cell retention control technology into SSB’s BIOSTAT® STR large-scale single-use bioreactors to create novel perfusion-enabled bioreactors. As a result of this collaboration, end users will stand to benefit from a single control system for 50 L to 2,000 L bioreactors used in perfusion cell culture applications. This single interface is designed to control cell growth, fluid management and cell retention in continuous and intensified bioprocessing and, ultimately, simplify the development and cGMP manufacture of biological drugs.
通过合作,赛多利斯Ste188体育手机版dim生物技术和Repligen将进一步合作,以装备SSB公司最近推出的ambr®250ht灌注单次使用小型生物反应器Repligen的KrosFlo®中空纤维过滤技术系统。生物反应器系统将SSB出售作为一个完整的单次使用的组件。这种优化设计节省了中空纤维过滤技术跨尺度,使客户能够快速发展轨道,并扩大其细胞培养灌注过程。
“赛多利斯Stedim Biotech公司不断扩大其集成组合的上游在过去的几年,重点放在强大,可扩展,自动化的单次使用的解决方案,用于高细胞密度应用而优化过。用Repligen的合作将导致易于实现,高性能和灌注生物反应器准备从工艺开发到商业化生产规模,”评论斯特凡Schlack,营销负责人SSB。
恭Gebski,在Repligen产品管理的副总裁说,“我们很高兴能与赛多利斯Stedim生物技术,生物反应器技术的全球创新合作伙伴。我们市场领先的XCELL™ATF控制技术的集成与SSB的高性能生物反应器提供的简化使灌注生物反应器解决方案为最终用户更快地开发细胞培养过程中,更有效地实现灌注“。
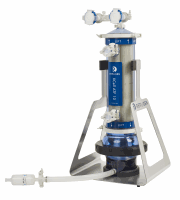
字幕:对于灌注细胞培养应用Repligen的XCELL™ATF细胞保持控制技术将被整合到SSB的BIOSTAT®STR大规模一次性生物反应器
对于高分辨率请点击图像。
字幕:Repligen XCELL™ATF细胞保持装置被示出为显著增加生物反应器的生产率;示出的是单次使用的XCELL™ATF 10
对于高分辨率请点击图像。
用于下载图像文件
照片链接BIOSTAT STR生物反应器
对于灌注细胞培养应用Repligen的XCELL™ATF细胞保持控制技术将被整合到SSB的BIOSTAT®STR大规模一次性生物反应器
照片链接Repligen XCELL™ATF
Repligen XCELL™ATF细胞保持装置被示出为显著增加生物反应器的生产率;示出的是单次使用的XCELL™ATF 10
照片链接AMBR 250ht灌注生物反应器
国家统计局最近发布的ambr®250ht灌注生物反应器系统配备了Repligen的KrosFlo®中空纤维过滤技术
关于赛多利斯Stedim生物技术
赛多利斯Stedim生物技术的产品和服务,使生物制药行业开发和生产药品安全有效地国际领先供应商。作为一个整体解决方案提供商,赛多利斯Stedim生物技术提供了涵盖生物制药生产的几乎所有步骤的组合。该公司专注于单一用途的技术和增值服务,以满足它所服务的行业的快速变化的技术需求。总部设在欧巴涅,法国,德国赛多利斯Stedim生物技术被引用于巴黎Euronext人才招聘999bottomen。凭借自身的制造和在欧洲,北美和亚洲R&d基地和销售公司的国际网络,赛多利斯Stedim生物技术具有全球影响力。2017年,该公司大约使用。5100名员工,并获得销售收入1,081.0亿€。
本新闻稿包含有关赛多利斯Stedim生物技术集团未来发展的声明。我们不能保证这些声明的内容将实际应用,因为这些陈述是基于假设和怀有一定的风险和不确定性的估计。
关于Repligen公司
Repligen公司(纳斯达克股票代码:RGEN)是一个全球性的生物加工公司,开发和销售,可提供成本和流程效率,以生物药品制造商全球高度创新的产品。The Company’s portfolio includes protein products (Protein A affinity ligands, cell culture growth factors), chromatography products (OPUS® pre-packed columns, chromatography resins, ELISA kits) and filtration products (including XCell™ ATF systems, TangenX™ Sius™ flat sheet TFF cassettes, and Spectrum KrosFlo™ hollow fiber TFF cartridges and systems). The XCell™ ATF Systems, available in stainless steel and single-use configurations, are used upstream to continuously eliminate waste from a bioreactor, to concentrate cells and increase product yield. Repligen’s corporate headquarters are in Waltham, MA (USA), with additional administrative and manufacturing operations in Shrewsbury, MA, Rancho Dominguez, CA, Lund, Sweden and Ravensburg, Germany.
下面根据1995年私人证券诉讼改革法案构成了一个“安全港”声明:本新闻稿包含前瞻性陈述,其已经依照1933年证券法第27A节的安全港条款,修订,和1934年证券交易法第21E条。Investors are cautioned that statements in this press release which are not strictly historical statements, including, without limitation, express or implied statements regarding Repligen’s partnership with Sartorius Stedim Biotech, the impact of a single control system on Repligen’s end users, and the future financial performance of Repligen constitute forward-looking statements identified by words like “believe,” “expect,” “may,” “will,” “should,” “seek,” “anticipate,” or “could” and similar expressions. Such forward-looking statements are subject to a number of risks and uncertainties that could cause actual results to differ materially from those anticipated, including, without limitation, risks associated with: our ability to successfully grow our bioprocessing business, including as a result of acquisition, commercialization or partnership opportunities; our ability to successfully integrate any acquisitions, our ability to develop and commercialize products and the market acceptance of our products; reduced demand for our products that adversely impacts our future revenues, cash flows, results of operations and financial condition; our ability to compete with larger, better financed bioprocessing, pharmaceutical and biotechnology companies; our compliance with all Food and Drug Administration and EMEA regulations; our volatile stock price; and other risks detailed in Repligen’s most recent Annual Report on Form 10-K on file with the Securities and Exchange Commission and the other reports that Repligen periodically files with the Securities and Exchange Commission. Actual results may differ materially from those Repligen contemplated by these forward-looking statements. These forward looking statements reflect management’s current views and Repligen does not undertake to update any of these forward-looking statements to reflect a change in its views or events or circumstances that occur after the date hereof except as required by law.
188bet体育滚球往来
赛多利斯Stedim生物技术
多米尼克·格罗内
企业传讯
赛多利斯企业管理有限公司
+49(0)551.308.3324
此邮件地址受spam bots保护。您必须启用浏览它。
Repligen
桑德拉·纽曼
高级主管投资者关系
+1 781-419-1881
此邮件地址受spam bots保护。您必须启用浏览它。
发贴者萨宾Duntze,B3C集团有限公司